TOP > Proprietary Technologies > Metal Heat Treatment > PVD Coating Treatment
PVD Coating Treatment
PVD Coating
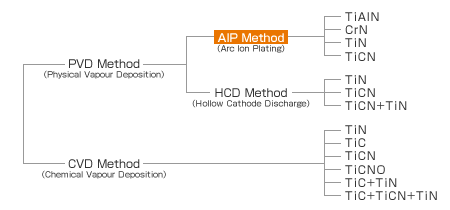
Physical vapor deposition (PVD) coating includes vacuum deposition, sputtering and ion plating. ONEX uses HCD ion plating machines and AIP ion plating machines for its PVD coating services.
Characteristics of the HCD Method
The hollow cathode (HCD) method first melts and vaporizes the raw material in a vacuum at between 400 and 500 degrees Celsius. The deposition substance is then ionized and combined with the gaseous body ionized in the same way. The resulting product is accelerated by cathode under glow discharge, and vapor deposited onto the surface of the fabricating material. The membrane formed during HCD-PVD coating retains superior smoothness, and is ideal for cutting tools as well as molds used in cold molding that require roughness.
Membrane Applications: TiN, TiCN, CrN
Characteristics of the AIP Method
The arc ion plating (AIP) method uses a vacuum arc discharge to vaporize the raw material. This then forms a membrane superior in terms of adhesiveness and whose raw material has a high ionization rate. In addition, an alloy membrane can be machined since the vaporizing source is not a fusion type. Generally the roughness of the AIP method is poorer than the HCD method due to the micro particles, but our AIP machine employs fine cathodes that enable it to improve surface roughness when compared to membranes in other AIP machines.
Membrane Applications: TiN, TiCN, TiAlN, CrN, etc.
The ONEX Advantage
Our HCD machine is able to accommodate comparatively large molds as it can perform treatments for parts that are up to 450 by 600 in size.
Our nitriding facilities control the atmosphere to produce nitriding without forming a compound layer. This means we act as a one-stop shop capable of accommodating even conventional parts that have been coated after removal of the compound layer following a nitriding treatment.
The Vacuum Department and Coating Department of the Atsugi Plant have acquired certification (approval no. 14BZ2900005) to manufacture medical device components and have accumulated a track record in QMS conformity studies.